EDTAs vs. Acetates: Maximizing Foliar Nutrient Spray Absorption for Higher Yields
Have you been using EDTA-chelated nutrients for your foliar sprays? It is commonplace to see EDTA products, created initially with soil application in mind, used in foliar nutrient sprays. Often, we apply foliar nutrients to improve plant growth and crop quality, appropriately manage the nutritional status of plants, enhance disease resistance, regulate nutrient deficiencies, and protect yield. However, research suggests that foliar applying nutrients in the EDTA form may only negatively affect our bottom line. In the following blog post, we will look at university and third-party research, learn what EDTAs and Acetates are, and discover the difference between the two.
The Acetate Advantage: What Makes Them Ideal for Foliar Nutrition
Iron Acetate Molecule - 173.93 g/mol
Acetates are derived from acetic acid, a compound made and used by plants. Because plants utilize acetic acid, acetates are something a plant can recognize more efficiently, which is part of the reason the plant more readily absorbs acetates.
Acetates form weaker bonds and fewer bonds with a nutrient. Weaker bonds allow for easier dissociation of the nutrient from the carrier, increasing absorption. Fewer bonds result in a smaller molecule size that can more easily pass through the outer protective layer of the leaf and be absorbed through the cuticle, cuticular irregularities, stomata, trichomes, or other epidermal structures to reach the internal cells of the plant. Smaller molecule size allows acetates to be absorbed more efficiently.
Superior Plant Recognition: Plants readily recognize and utilize acetates, leading to faster and more efficient nutrient absorption.
Weaker Bonds: The weaker bonds in acetates allow nutrients to dissociate easily from the carrier molecule, enhancing uptake by the plant.
Smaller Molecule Size: Acetate molecules are significantly smaller than EDTA molecules, facilitating their passage through the tiny pores on leaf surfaces.
The EDTA Dilemma: Why It's Not Always the Best Choice for Foliar Sprays
EDTA Molecule - 435 g/mol
EDTA (ethylenediaminetetraacetic acid) fertilizers contain nutrients that are chelated. The term Chelation is derived from the Greek word chele, which means claw. ETDAs bond to metal ions and tightly lock onto them. ETDAs in medicine are used as a treatment for heavy metal toxicity. They are intravenously injected into a patient, whereupon the EDTAs tightly lock in the heavy metal, preventing interaction and allowing the metal to pass through the body harmlessly.
In agriculture, EDTAs protect nutrients from soil or nutrient interaction. This practice of nutrient chelation, when utilized for soil application, works effectively. However, when nutrients EDTAS are foliar applied, their greatest benefit becomes their biggest weakness.
Large Molecule Size: EDTA molecules are bulky, making it difficult for them to penetrate leaf pores. For example, the molecule size of iron ETDA vs. iron acetate as seen above in. The iron acetate molecule size is 173.93 grams per mole, while the iron EDTA is around 435 grams per mole. That means the ETDA molecule size is around 2 1/2 times larger than an acetate's.
Strong Bonds: The tight grip of EDTA on nutrients makes it challenging for plants to access and utilize them efficiently. EDTA molecules prevent reactivity due to nutrients being tightly held. While this prevents nutrient interaction, it hinders absorption. In actuality, the use of EDTAs in nutrient sprays is more beneficial for the manufacturer (so they can mix multiple nutrients without fear of interaction), than they are for the grower.
So, how much does the size and lack of activity hurt absorption? Those who regularly use EDTA products in foliar sprays may still be weary. Still, University research from the University of Arkansas and third-party research may shock you.
The University of Arkansas Research: Acetates Outperform EDTAs in Foliar Absorption
A team at the University of Arkansas, consisting of Samuel Stacey and Derrick Oosterhuis, conducted a study to test the efficacy of EDTA forms of iron and zinc compared to other forms of these elements. Their research found that the absorption rates for iron and zinc in the EDTA form were significantly lower than those of other forms. The researchers suggested that the EDTA molecules might have competed with the aqueous pores of leaf foliage, hindering absorption.
The key finding from this study highlighted, "This study showed that EDTA should not be used in trace element foliar sprays, particularly given the high cost of these chelates to farmers." Despite this, the study also noted that while EDTA is not suitable for foliar application, it remains a viable option for soil application.
Third-Party Research Data
CultivAce partnered with a third-party research organization to test the efficacy of nutrient foliar sprays. The trials included three products: zinc acetate (ZnAce), manganese acetate (MnAce), and iron acetate (FeAce). They included four repetitions of multiple products in a randomized block design. These repetitions were then averaged.
Manganese Acetate v. EDTA
In this 2024 trial, the efficacy of manganese foliar spray was tested. Three products were applied in multiple repetitions: manganese acetate, manganese amino acid chelate, and manganese EDTA.
Manganese acetate increased foliage manganese levels by 29.28% over manganese amino acid chelate. Manganese acetate raised foliar manganese levels by 31.16% over the manganese EDTA.
The manganese acetate raised foliage manganese levels by 46.29% over the untreated check. Manganese acetate by fair and large was the best-performing treatment in this trial. If you were facing manganese deficiency what product would you choose?
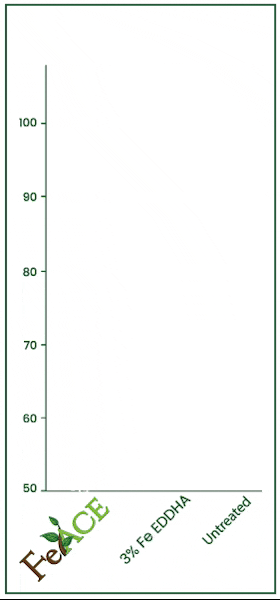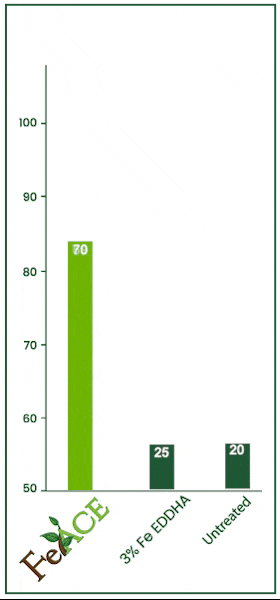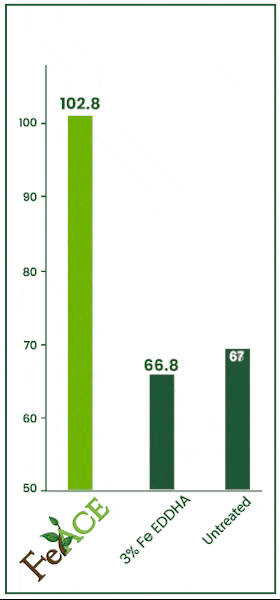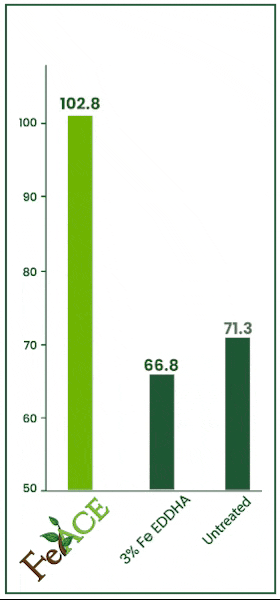
Iron Acetate v. EDDHA
In this 2024 trial, the efficacy of iron foliar sprays was tested. Iron acetate and iron EDHHA were applied at two quarts per acre over multiple repetitions. FeAce iron acetate increased foliage iron levels by 53.89% over the iron EDHHA.
FeAce iron acetate increased foliar iron levels by 44.17% over the untreated check. Often, EDDHA is primarily soil applied. However, many iron EDHHA products are labeled for foliar application as well.
Essentially, foliar applying iron EDHHA would be akin to applying no product whatsoever.
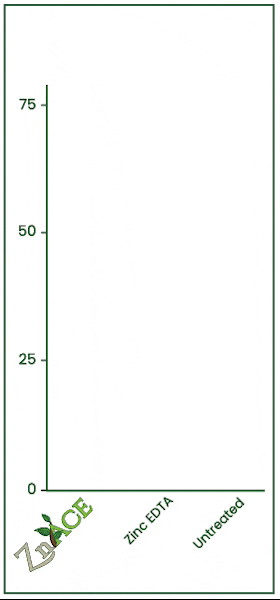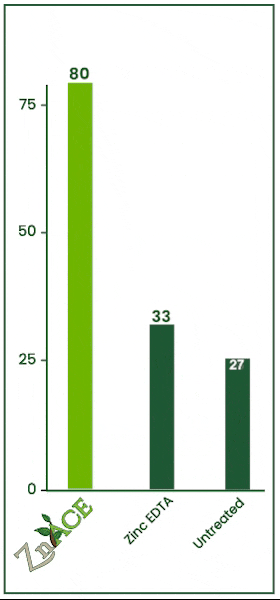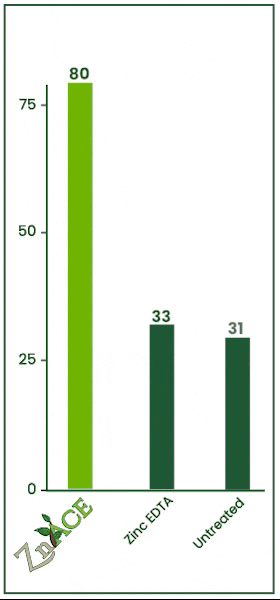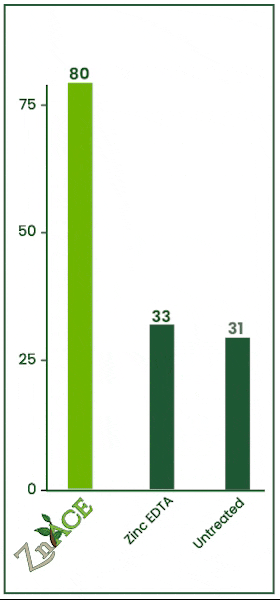
Zinc Acetate v. EDTA
In this 2023 trial, the efficacy of zinc foliar sprays was tested against zinc EDTA. Both products were applied at 2 quarts per acre. Zn Ace zinc acetate increased foliage zinc levels by 47 ppm over the zinc edta product.
ZnAce zinc acetate increased tissue zinc levels by 49 ppm over the untreated check. Zinc acetate vastly outperformed EDTA in this trial.
The untreated check and EDTA had no statistically significant difference meaning in this trial, from an economic standpoint, it would have been better to apply no product than to apply an EDTA.
Conclusion
The biggest takeaway from this blog post for you, the grower, should be to avoid EDTA foliar sprays at all costs. EDTA nutrients have lower absorption rates due to their large size and the many bonds that tightly lock in a nutrient.
When we apply EDTA nutrient chelation, we rob our crops of necessary nutrition and diminish our profits in the process.
For those interested in learning more, CultivAce president and founder Wayne Sledge recently participated in a Webinar that, in greater depth, described EDTAS, Acetates, and soil/nutrient interactions that can prevent uptake. The webinar can be found here.
Another valuable topic covered in the webinar but that didn’t fit in appropriately here is the Mulder’s chart. The Mulder’s chart shows the effect nutrient applications have on each other. The Mulder’s chart can be found here and to my knowledge is the only interactive version currently available.
Further Resources:
The University of Arkansas can be found here